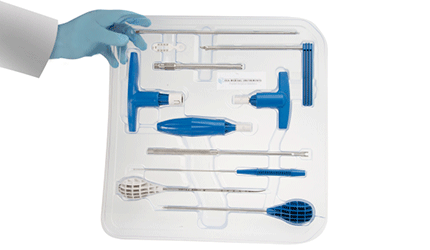
ECA Medical Gets FDA OK for Disposable Spine Implant Instruments
ECA Medical Instruments plans to launch its Intelligent Implant Systems Revolution Spinal System during the 2015 second quarter, following the receipt of FDA clearance for its fully disposable spine implant fixation instrument kit. The kit, an industry first, will be used with the IIS system.
The instruments — packaged in a single, sterilized tray — include cannulated torque-limiters, ratchets and fixed drivers that are used by surgeons to perform open and minimally invasive spinal surgeries. Combining the implants and instruments in a single set helps to increase productivity and accurate fixation, while reducing operational costs and risk of infection, ECA says.
All of the instruments are disposable and biodegradable, eliminating the need to sterilize them prior to each procedure, therefore minimizing the risk of cross contamination, the company adds. — Kellen Owings
